
HL Paper 2
Cobalt forms the transition metal complex [Co(NH3)4 (H2O)Cl]Br.
Trends in physical and chemical properties are useful to chemists.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
State the shape of the complex ion.
Deduce the charge on the complex ion and the oxidation state of cobalt.
Describe, in terms of acid-base theories, the type of reaction that takes place between the cobalt ion and water to form the complex ion.
Markscheme
Any three of:
Group 1:
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and nucleus for M2.
Accept “weaker metallic bonds” for M2.
Group 17:
number of electrons/surface area/molar mass increase
London/dispersion/van der Waals’/vdw forces increase
Accept “atomic mass” for “molar mass”.
[Max 3 Marks]
«distorted» octahedral
Accept “square bipyramid”.
Charge on complex ion: 1+/+
Oxidation state of cobalt: +2
Lewis «acid-base reaction»
H2O: electron/e– pair donor
OR
Co2+: electron/e– pair acceptor
Examiners report
An equation for the combustion of propane is given below.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
Determine the standard enthalpy change, , for this reaction, using section 11 of the data booklet.
Calculate the standard enthalpy change, , for this reaction using section 12 of the data booklet.
Predict, giving a reason, whether the entropy change, , for this reaction is negative or positive.
Calculate for the reaction in , using section 12 of the data booklet.
The standard molar entropy for oxygen gas is .
Calculate the standard Gibbs free energy change, , in , for the reaction at 5 °C, using your answers to (b) and (d). Use section 1 of the data booklet.
(If you did not obtain an answer to (b) or (d) use values of and respectively, although these are not the correct answers.)
Markscheme
Bonds broken: 8(C–H) + 2(C–C) + 5(O=O) / 8 × 414 «kJ mol−1» + 2 × 346 «kJ mol−1» + 5 × 498 «kJ mol−1» / 6494 «kJ» ✔
Bonds formed: 6(C=O) + 8(O–H) / 6 × 804 «kJ mol−1» + 8 × 463 «kJ mol−1» / 8528 «kJ» ✔
«Enthalpy changebonds brokenbonds formed ✔
Award [3] for correct final answer.
AND AND ✔
✔
Award [2] for correct final answer.
Award [1 max] for .
positive AND more moles «of gas» in products ✔
AND AND AND ✔
✔
Award [2] for correct final answer.
✔
✔
Award [2] for correct final answer.
Examiners report
Many candidates had difficulty determining the number and type of bonds broken or formed and consequently this was the part of question 3 that was most poorly attempted. Those that could identify these bonds performed the calculations correctly.
Enthalpy calculations using enthalpy of formation data were generally well done.
Most knew that entropy increased however some lost the mark by not including an explanation based on increase number of mol of gaseous products.
Calculating ΔSө, like most other calculations, was well done.
ΔGө calculations were also well done, with some not seeing that specific units were to be used.
Ammonia is produced by the Haber–Bosch process which involves the equilibrium:
N2 (g) + 3 H2 (g) 2 NH3 (g)
The percentage of ammonia at equilibrium under various conditions is shown:
[The Haber Bosch Process [graph] Available at: https://commons.wikimedia.org/wiki/File:Ammonia_yield.png
[Accessed: 16/07/2022].]
One factor affecting the position of equilibrium is the enthalpy change of the reaction.
The standard free energy change, ΔG⦵, for the Haber–Bosch process is –33.0 kJ at 298 K.
Deduce the expression for the equilibrium constant, Kc, for this equation.
State how the use of a catalyst affects the position of the equilibrium.
With reference to the reaction quotient, Q, explain why the percentage yield increases as the pressure is increased at constant temperature.
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
Outline why the value obtained in (b)(i) might differ from a value calculated using ΔHf data.
Demonstrate that your answer to (b)(i) is consistent with the effect of an increase in temperature on the percentage yield, as shown in the graph.
State, giving a reason, whether the reaction is spontaneous or not at 298 K.
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
Calculate the entropy change for the Haber–Bosch process, in J mol–1 K–1 at 298 K. Use your answer to (b)(i) and section 1 of the data booklet.
Outline, with reference to the reaction equation, why this sign for the entropy change is expected.
Markscheme
✔
same/unaffected/unchanged ✔
increasing pressure increases «all» concentrations
OR
increasing pressure decreases volume ✔
Q becomes less than Kc
OR
affects the lower line/denominator of Q expression more than upper line/numerator ✔
«for Q to once again equal Kc,» ratio of products to reactants increases
OR
«for Q to once again equal Kc,» equilibrium shifts to right/products ✔
Award [2 max] for answers that do not refer to Q.
bonds broken: N≡N + 3(H-H) /«1 mol×»945 «kJ mol–1» + 3«mol»×436 «kJ mol–1» / 945 «kJ» + 1308 «kJ» / 2253 «kJ» ✔
bonds formed: 6(N-H) / 6«mol»×391 «kJ mol–1» / 2346 «kJ» ✔
ΔH = «2253 kJ - 2346 kJ = » -93 «kJ» ✔
Award [2 max] for (+)93 «kJ».
«N-H» bond enthalpy is an average «and may not be the precise value in NH3» ✔
Accept ΔHf data are more accurate / are not average values.
increased temperature decreases yield «as shown on graph» ✔
shifts equilibrium in endothermic/reverse direction ✔
spontaneous AND ΔG < 0 ✔
✔
✔
Award [2] for correct final answer.
Accept answers in the range 4.4×105 to 6.2×105 (arises from rounding of ln K).
ΔG = «ΔH – TΔS =» –93000 «J» – 298«K» × ΔS = –33000 ✔
ΔS = 〈〈〉〉 = –201 «J mol–1 K–1» ✔
Do not penalize failure to convert kJ to J in both (c)(ii) and (c)(iii).
Award [2] for correct final answer
Award [1 max] for (+) 201 «J mol–1 K–1».
Award [2] for –101 or –100.5 «J mol–1 K–1».
«forward reaction involves» decrease in number of moles «of gas» ✔
Examiners report
Deducing the equilibrium constant expression for the given equation was done very well.
Good performance; however, some misread the question as asking for the effect of a catalyst on equilibrium, rather than on the position of equilibrium.
Mediocre performance; very few identified the effect of increasing pressure on all concentrations. Consequently, Q becomes less than Kc (it affects the denominator of Q expression more than the numerator) was not addressed. Question was often answered with respect to kinetics, namely greater frequency of collisions and speed of reaction rather than from equilibrium perspective based on effect of increase in pressure on concentrations.
Good performance; often the bond energy for single N–N bond instead of using it for the triple bond and not taking into consideration the coefficient of three in calculation of bond enthalpies of ammonia. Also, instead of using BE of bonds broken minus those that were formed, the operation was often reversed. Students should be encouraged to draw the Lewis structures in the equations first to determine the bonds being broken and formed.
Outlining why ΔHrxn based on BE values differ due to being average compared to using ΔHf values was generally done well.
Good performance; some did not relate that increased temperature decreases yield «as shown on graph» and others arrived at incorrect shift in equilibrium for the reaction.
Reason for the reaction being spontaneous was generally very done well indeed.
Good performance; for lnK calculation in the equation ΔG = RTlnK, ΔG unit had to be converted from kJ to J. This led to an error of 1000 in the value of lnK for some.
Very good performance; since the unit for S is J mol˗1 K˗1, ΔG and ΔH needed to be converted from kJ to J, but that was not done in some cases.
Average performance for sign of the entropy change expected for the reaction. Some answers were based on ΔG value rather than in terms of decrease in number of moles of gas or had no idea how to address the question.
Oxygen exists as two allotropes, diatomic oxygen, O2, and ozone, O3.
Draw a Lewis (electron dot) structure for ozone.
Discuss the relative length of the two O−O bonds in ozone.
Explain why there are frequencies of UV light that will dissociate O3 but not O2.
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
Markscheme
✔
Accept any combination of lines, dots or crosses to represent electrons.
Do not accept structures that represent 1.5 bonds.
both equal ✔
delocalization/resonance ✔
Accept bond length between 121 and 148 pm/ that of single O−O bond and double O=O bond for M1.
bond in O3 is weaker
OR
O3 bond order 1.5/< 2 ✔
Do not accept bond in O3 is longer for M1.
lower frequency/longer wavelength «UV light» has enough energy to break the O–O bond in O3 «but not that in O2» ✔
Accept “lower frequency/longer wavelength «UV light» has lower energy”.
✔
AND
✔
Do not penalize missing radical.
Accept:for M2:
AND
Examiners report
Carbon forms many compounds.
C60 and diamond are allotropes of carbon.
Chlorine reacts with methane.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Outline two differences between the bonding of carbon atoms in C60 and diamond.
Explain why C60 and diamond sublime at different temperatures and pressures.
State two features showing that propane and butane are members of the same homologous series.
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
Draw the full structural formula of (Z)-but-2-ene.
Write the equation for the reaction between but-2-ene and hydrogen bromide.
State the type of reaction.
Suggest two differences in the 1H NMR of but-2-ene and the organic product from (d)(ii).
Predict, giving a reason, the major product of reaction between but-1-ene and steam.
Explain the mechanism of the reaction between 1-bromopropane, CH3CH2CH2Br, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
Deduce the splitting pattern in the 1H NMR spectrum for 1-bromopropane.
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
Draw and label an enthalpy level diagram for this reaction.
Markscheme
Any two of:
C60 fullerene: bonded to 3 C AND diamond: bonded to 4 C ✔
C60 fullerene: delocalized/resonance AND diamond: not delocalized / no resonance ✔
C60 fullerene: sp2 AND diamond: sp3 ✔
C60 fullerene: bond angles between 109–120° AND diamond: 109° ✔
Accept "bonds in fullerene are shorter/stronger/have higher bond order OR bonds in diamond longer/weaker/have lower bond order".
diamond giant/network covalent AND sublimes at higher temperature ✔
C60 molecular/London/dispersion/intermolecular «forces» ✔
Accept “diamond has strong covalent bonds AND require more energy to break «than intermolecular forces»” for M1.
same general formula / CnH2n+2 ✔
differ by CH2/common structural unit ✔
Accept "similar chemical properties".
Accept “gradation/gradual change in physical properties”.
ALTERNATIVE 1:
Test:
add bromine «water»/Br2 (aq) ✔
Result:
«orange/brown/yellow» to colourless/decolourised ✔
Do not accept “clear” for M2.
ALTERNATIVE 2:
Test:
add «acidified» KMnO4 ✔
Result:
«purple» to colourless/decolourised/brown ✔
Accept “colour change” for M2.
ALTERNATIVE 3:
Test:
add iodine / ✔
Result:
«brown» to colourless/decolourised ✔
Accept
CH3CH=CHCH3 + HBr (g) → CH3CH2CHBrCH3
Correct reactants ✔
Correct products ✔
Accept molecular formulas for both reactants and product
«electrophilic» addition/EA ✔
Do not accept nucleophilic or free radical addition.
ALTERNATIVE 1: Any two of:
but-2-ene: 2 signals AND product: 4 signals ✔
but-2-ene: «area ratio» 3:1/6:2 AND product: «area ratio» 3:3:2:1 ✔
product: «has signal at» 3.5-4.4 ppm «and but-2-ene: does not» ✔
but-2-ene: «has signal at» 4.5-6.0 ppm «and product: does not» ✔
ALTERNATIVE 2:
but-2-ene: doublet AND quartet/multiplet/4 ✔
product: doublet AND triplet AND quintet/5/multiplet AND sextet/6/multiplet ✔
Accept “product «has signal at» 1.3–1.4 ppm «and but-2-ene: does not»”.
CH3CH2CH(OH)CH3 ✔
«secondary» carbocation/CH3CH2CH+CH3 more stable ✔
Do not accept “Markovnikov’s rule” without reference to carbocation stability.
curly arrow going from lone pair/negative charge on O in HO– to C ✔
curly arrow showing Br breaking ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
formation of organic product CH3CH2CH2OH AND Br– ✔
Do not allow curly arrow originating on H in HO–.
Accept curly arrow either going from bond between C and Br to Br in 1-bromopropane or in the transition
state.
Do not penalize if HO and Br are not at 180° to each other.
Award [3 max] for SN1 mechanism.
triplet/3 AND multiplet/6 AND triplet/3 ✔
bond breaking: C–H + Cl–Cl / 414 «kJ mol–1» + 242 «kJ mol–1»/656 «kJ»
OR
bond breaking: 4C–H + Cl–Cl / 4 × 414 «kJ mol–1» + 242 «kJ mol–1» / 1898 «kJ» ✔
bond forming: «C–Cl + H–Cl / 324 kJ mol–1 + 431 kJ mol–1» / 755 «kJ»
OR
bond forming: «3C–H + C–Cl + H–Cl / 3 × 414 «kJ mol–1» + 324 «kJ mol–1» + 431 kJ mol–1» / 1997 «kJ» ✔
«ΔH = bond breaking – bond forming = 656 kJ – 755 kJ» = –99 «kJ» ✔
Award [3] for correct final answer.
Award [2 max] for 99 «kJ».
reactants at higher enthalpy than products ✔
ΔH/-99 «kJ» labelled on arrow from reactants to products
OR
activation energy/Ea labelled on arrow from reactant to top of energy profile ✔
Accept a double headed arrow between reactants and products labelled as ΔH for M2.
Examiners report
A challenging question, requiring accurate knowledge of the bonding in these allotropes (some referred to graphite, clearly the most familiar allotrope). The most frequent (correct) answer was the difference in number of bonded C atoms and hybridisation in second place. However, only 30% got a mark.
Again, this was a struggle between intermolecular forces and covalent bonds and this proved to be even harder than (a)(i) with only 25% of candidates getting full marks. The distinction between giant covalent/covalent network in diamond and molecular in C60 and hence resultant sublimation points, was rarely explained. There were many general and vague answers given, as well as commonly (incorrectly) stating that intermolecular forces are present in diamond. As another example of insufficient attention to the question itself, many candidates failed to say which would sublime at a higher temperature and so missed even one mark.
This easy question was quite well answered; same/similar physical properties and empirical formula were common errors.
Candidates misinterpreted the question and mentioned CH3+, i.e., the lost fragment; the other very common error was -COOH which shows a complete lack of understanding of MS considering the question is about butane so O should never appear.
Well answered by most, but some basic chemistry was missing when reporting results, perhaps as a result of little practical work due to COVID. A significant number suggested IR spectrometry, very likely because the question followed one on H NMR spectroscopy, thus revealing a failure to read the question properly (which asks for a test). Some teachers felt that adding "chemical" would have avoided some confusion.
Most were able to draw this isomer correctly, though a noticeable number of students included the Z as an atom in the structural formula, showing they were completely unfamiliar with E/Z notation.
Well done in general and most candidates wrote correct reagents, eventually losing a mark when considering H2 to be a product alongside 2-bromobutane.
Very well answered, some mentioned halogenation which is a different reaction.
A considerable number of students (40%) got at least 1 mark here, but marks were low (average mark 0.9/2). Common errors were predicting 3 peaks, rather than 4 for 2 -bromobutane and vague / unspecific answers, such as ‘different shifts’ or ‘different intensities’. It is surprising that more did not use H NMR data from the booklet; they were not directed to the section as is generally done in this type of question to allow for more general answers regarding all information that can be obtained from an H NMR spectrum.
Product was correctly predicted by many, but most used Markovnikov's Rule to justify this, failing to mention the stability of the secondary carbocation, i.e., the chemistry behind the rule.
As usual, good to excellent candidates (47.5%) were able to get 3/4 marks for this mechanism, while most lost marks for carelessness in drawing arrows and bond connectivity, issues with the lone pair or negative charge on the nucleophile, no negative charge on transition state, or incorrect haloalkane. The average mark was thus 1.9/4.
Another of the very poorly answered questions where most candidates (90%) failed to predict 3 peaks and when they did, considered there would be a quartet instead of multiplet/sextet; other candidates seemed to have no idea at all. This is strange because the compound is relatively simple and while some teachers considered that predicting a sextet may be beyond the current curriculum or just too difficult, they could refer to a multiplet; a quartet is clearly incorrect.
Only the very weak candidates were unable to calculate the enthalpy change correctly, eventually missing 1 mark for inverted calculations.
Most candidates drew correct energy profiles, consistent with the sign of the energy change calculated in the previous question. And again, only very weak candidate failed to get at least 1 mark for correct profiles.
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Now consider the second stage of the reaction.
CO (g) + 2H2 (g) CH3OH (l) ΔH⦵ = –129 kJ
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.
The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
State one reason why you would expect the value of ΔH calculated from the values, given in section 12 of data booklet, to differ from your answer to (d)(i).
State the expression for Kc for this stage of the reaction.
State and explain the effect of increasing temperature on the value of Kc.
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
Justify the sign of ΔS with reference to the equation.
Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the spontaneity of the reaction.
Markscheme
curve higher AND to left of T1 ✔
new/catalysed Ea marked AND to the left of Ea of curve T1 ✔
Do not penalize curve missing a label, not passing exactly through the origin, or crossing x-axis after Ea.
Do not award M1 if curve drawn shows significantly more/less molecules/greater/smaller area under curve than curve 1.
Accept Ea drawn to T1 instead of curve drawn as long as to left of marked Ea.
methanoic acid/HCOOH/CHOOH
OR
methanal/HCHO ✔
Accept “carbon dioxide/CO2”.
CH4(g) + H2O(g) CH3OH(l) + H2(g) ✔
Accept arrow instead of equilibrium sign.
amount of methane = « = » 0.498 «mol» ✔
amount of hydrogen = amount of methane / 0.498 «mol» ✔
volume of hydrogen = «0.498 mol × 22.7 dm3 mol−1 = » 11.3 «dm3» ✔
Award [3] for final correct answer.
Award [2 max] for 11.4 «dm3 due to rounding of mass to 16/moles to 0.5. »
Σbonds broken = 4 × 414 «kJ» + 2 × 463 «kJ» / 2582 «kJ» ✔
Σbonds formed = 1077 «kJ» + 3 × 436 «kJ» / 2385 «kJ» ✔
ΔH «= Σbonds broken − Σbonds formed =( 2582 kJ − 2385 kJ)» = «+»197«kJ» ✔
Award [3] for final correct answer.
Award [2 Max] for final answer of −197 «kJ»
bond energies are average values «not specific to the compound» ✔
✔
Kc increases AND «forward» reaction endothermic ✔
«ΔG⦵ = − RT lnKc»
ΔG⦵ = − 8.31 «J K−1 mol−1» × 298 «K» × ln (1.01) / −24.6 «J mol−1» ✔
= −0.0246 «kJ mol–1» ✔
Award [2] for correct final answer.
Award [1 max] for +0.0246 «kJ mol–1».
«ΔG⦵ = ΔH⦵ − TΔS⦵»
ΔG⦵ = −129 «kJ mol–1» − (298 «K» × ΔS) = −0.0246 «kJ mol–1» ✔
ΔS⦵ = « = » −433 «J K–1 mol–1» ✔
Award [2] for correct final answer.
Award [1 max] for “−0.433 «kJ K–1 mol–1»”.
Award [1 max] for “433” or “+433” «J K–1 mol–1».
Award [2] for −430 «J K–1 mol–1» (result from given values).
«negative as» product is liquid and reactants gases
OR
fewer moles of gas in product ✔
reaction «more» spontaneous/ΔG negative/less positive AND effect of negative entropy decreases/TΔS increases/is less negative/more positive
OR
reaction «more» spontaneous/ΔG negative/less positive AND reaction exothermic «so Kc increases » ✔
Award mark if correct calculation shown.
Examiners report
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ.
Predict, giving a reason, which product is the most stable.
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Deduce the splitting pattern you would expect for the signals in a high resolution 1H NMR spectrum.
2.3 ppm:
9.8 ppm:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
Markscheme
C2H2 (g) + 2.5O2 (g) → 2CO2 (g) + H2O (l)
OR
2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O (l) [✔]
[✔]
Note: Accept any valid combination of lines, dots and crosses.
«ethyne» shorter AND a greater number of shared/bonding electrons
OR
«ethyne» shorter AND stronger bond [✔]
London/dispersion/instantaneous dipole-induced dipole forces [✔]
ethanal [✔]
«sum of bond enthalpies of reactants =» 2(C—H)+C ≡ C + 2(O—H)
OR
2 × 414 «kJ mol-1» + 839 «kJ mol-1» + 2 × 463 «kJ mol-1»
OR
2593 «kJ» [✔]
«sum of bond enthalpies of A =» 3(C—H) + C=C + C—O + O—H
OR
3 × 414 «kJ mol-1» + 614 «kJ mol-1» + 358 «kJ mol-1» + 463 «kJ mol-1»
OR
2677 «kJ» [✔]
«enthalpy of reaction = 2593 kJ – 2677 kJ» = –84 «kJ» [✔]
Note: Award [3] for correct final answer.
B AND it has a more negative/lower enthalpy/«potential» energy
OR
B AND more exothermic «enthalpy of reaction from same starting point» [✔]
Identity of product: «B»
IR spectrum:
1700–1750 «cm–1 band» AND carbonyl/CO group present
OR
no «band at» 1620–1680 «cm–1» AND absence of double bond/C=C
OR
no «broad band at» 3200–3600 «cm–1 » AND absence of hydroxyl/OH group [✔]
1H NMR spectrum:
«only» two signals AND A would have three
OR
«signal at» 9.4–10.0 «ppm» AND «H atom/proton of» aldehyde/–CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of alkyl/CH next to» aldehyde/CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of» RCOCH2- present
OR
no «signal at» 4.5–6.0 «ppm» AND absence of «H atom/proton next to» double bond/C=C ✔
Note: Accept a specific value or range of wavenumbers and chemical shifts.
Accept “two signals with areas 1:3”.
2.3 ppm: doublet [✔]
9.8 ppm: quartet [✔]
Reagents:
acidified/H+ AND «potassium» dichromate«(VI)»/K2Cr2O7/Cr2O72- [✔]
Conditions:
distil «the product before further oxidation» [✔]
Note: Accept “«acidified potassium» manganate(VII)/KMnO4/MnO4-/permanganate”.
Accept “H2SO4” or “H3PO4” for “H+”.
Accept “more dilute dichromate(VI)/manganate(VII)” or “excess ethanol”.
Award M1 if correct reagents given under “Conditions”.
–1 [✔]
Any three of:
has an oxygen/O atom with a lone pair [✔]
that can form hydrogen bonds/H-bonds «with water molecules» [✔]
hydrocarbon chain is short «so does not disrupt many H-bonds with water molecules» [✔]
«large permanent» dipole-dipole interactions with water [✔]
Examiners report
All candidates were able to write the correct reactants/products for combustion of ethyne, but a few failed to balance correctly.
Most drew correct Lewis structures for ethyne, though some drew ethene.
Surprisingly very few explained the difference in bond length/strength looking at electrons shared and just gave the shorter/triple or longer/single bond answer.
Good to see that most candidates identified the specific IMF correctly.
Most candidates gave the correct IUPAC name.
Candidates were able to calculate the ΔH of the given reaction correctly; a few inverted the calculations or made mathematical errors.
Generally well done, most common error was stating that the enthalpy change was “larger” without the indication that it was an exothermic change or the sign.
Interpretation of spectra was very good and the few candidates that lost marks with 1H NMR data rather than IR, for example simply mentioning two signals for B. However, most candidates that attempted this question got full marks.
The stronger candidates were able to predict the splitting pattern correctly, others inverted the answer, but many others repeated the information for protons with the given chemical shift, which is unexpected since wording was straightforward.
Candidates seemed to be confused by the prompts, reagent and conditions, so often included the acid among conditions. Careless errors were common such as the wrong charge on the dichromate ion. Few candidates suggest permanganate as an option.
Most candidates were able to calculate oxidation state of carbon in B.
Candidates did not understand that they must mention the IMF responsible for the solubility. Most candidates explained the polarity of the aldehyde and water but did not mention that this results in permanent dipole-dipole interactions; many did mention H-bonding. The mention of the lone pair on O atom and short hydrocarbon chain were very rare.
This question is about ethene, C2H4, and ethyne, C2H2.
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
Ethyne reacts with chlorine in a similar way to ethene. Formulate equations for the following reactions.
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHΘ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
Explain, giving two reasons, the difference in the values for (c)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
One possible Lewis structure for benzene is shown.

State one piece of physical evidence that this structure is incorrect.
Markscheme
nickel/Ni «catalyst»
high pressure
OR
heat
Accept these other catalysts: Pt, Pd, Ir, Rh, Co, Ti.
Accept “high temperature” or a stated temperature such as “150 °C”.
[2 marks]

Ignore square brackets and “n”.
Connecting line at end of carbons must be shown.
[1 mark]
ethyne: C2H2 + Cl2 → CHClCHCl
benzene: C6H6 + Cl2 → C6H5Cl + HCl
Accept “C2H2Cl2”.
[2 marks]
ΔHΘ = bonds broken – bonds formed
«ΔHΘ = 3(C≡C) – 6(CC)benzene / 3 839 – 6 507 / 2517 – 3042 =» –525 «kJ»
Award [2] for correct final answer.
Award [1 max] for “+525 «kJ»”.
Award [1 max] for:
«ΔHΘ = 3(C≡C) – 3(C–C) – 3(C=C) / 3 839 – 3 346 – 3 614 / 2517 – 2880 =» –363 «kJ».
[2 marks]
ΔHΘ = ΣΔHf (products) – ΣΔHf (reactants)
«ΔHΘ = 49 kJ – 3 228 kJ =» –635 «kJ»
Award [2] for correct final answer.
Award [1 max] for “+635 «kJ»”.
[2 marks]
ΔHf values are specific to the compound
OR
bond enthalpy values are averages «from many different compounds»
condensation from gas to liquid is exothermic
Accept “benzene is in two different states «one liquid the other gas»” for M2.
[2 marks]
«ΔSΘ = 173 – 3 201 =» –430 «J K–1»
[1 mark]
T = «25 + 273 =» 298 «K»
ΔGϴ «= –635 kJ – 298 K × (–0.430 kJ K–1)» = –507 kJ
ΔGϴ < 0 AND spontaneous
ΔGϴ < 0 may be inferred from the calculation.
[3 marks]
equal C–C bond «lengths/strengths»
OR
regular hexagon
OR
«all» C–C have bond order of 1.5
OR
«all» C–C intermediate between single and double bonds
Accept “all C–C–C bond angles are equal”.
[1 mark]
Examiners report
The photochemical chlorination of methane can occur at low temperature.
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
Markscheme
«ΔH θ =» –82.0 «kJ» –92.3 «kJ» – (–74.0 «kJ»)
«ΔH θ =» –100.3 «kJ»
Award [2] for correct final answer.
[2 marks]
Examiners report
Soluble acids and bases ionize in water.
A solution containing 0.510 g of an unknown monoprotic acid, HA, was titrated with 0.100 mol dm–3 NaOH(aq). 25.0 cm3 was required to reach the equivalence point.
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
State a technique other than a pH titration that can be used to detect the equivalence point.
Deduce the pKa for this acid.
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
Markscheme
weak AND pH at equivalence greater than 7
OR
weak acid AND forms a buffer region
[1 mark]
calorimetry
OR
measurement of heat/temperature
OR
conductivity measurement
Accept “indicator” but not “universal indicator”.
[1 mark]
«pKa = pH at half-equivalence =» 5.0
[1 mark]
Ka =
[H3O+] = «mol dm–3»
pH = «» 3.57
Award [3] for correct final answer to two decimal places.
If quadratic equation used, then: [H3O+] = 2.459 × 10–4 «mol dm–3» and pH = 3.61
[3 marks]
Examiners report
Limestone can be converted into a variety of useful commercial products through the lime cycle. Limestone contains high percentages of calcium carbonate, CaCO3.
Thermodynamic data for the decomposition of calcium carbonate is given.
The second step of the lime cycle produces calcium hydroxide, Ca(OH)2.
Calcium hydroxide reacts with carbon dioxide to reform calcium carbonate.
Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O (l)
Calcium carbonate is heated to produce calcium oxide, CaO.
CaCO3 (s) → CaO (s) + CO2 (g)
Calculate the volume of carbon dioxide produced at STP when 555 g of calcium carbonate decomposes. Use sections 2 and 6 of the data booklet.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
Calculate the change in entropy, ΔS, in J K−1, for the decomposition of calcium carbonate.
Determine the temperature, in K, at which the decomposition of calcium carbonate becomes spontaneous, using b(i), b(ii) and section 1 of the data booklet.
(If you do not have answers for b(i) and b(ii), use ΔH = 190 kJ and ΔS = 180 J K−1, but these are not the correct answers.)
Sketch an energy profile for the decomposition of calcium carbonate based on your answer to b(i), labelling the axes and activation energy, Ea.
State how adding a catalyst to the reaction would impact the enthalpy change of reaction, ΔH, and the activation energy, Ea.
Write the equation for the reaction of Ca(OH)2 (aq) with hydrochloric acid, HCl (aq).
Determine the volume, in dm3, of 0.015 mol dm−3 calcium hydroxide solution needed to neutralize 35.0 cm3 of 0.025 mol dm−3 HCl (aq).
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
Determine the mass, in g, of CaCO3 (s) produced by reacting 2.41 dm3 of 2.33 × 10−2 mol dm−3 of Ca(OH)2 (aq) with 0.750 dm3 of CO2 (g) at STP.
2.85 g of CaCO3 was collected in the experiment in d(i). Calculate the percentage yield of CaCO3.
(If you did not obtain an answer to d(i), use 4.00 g, but this is not the correct value.)
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
Markscheme
«nCaCO3 = =» 5.55 «mol» ✓
«V = 5.55 mol × 22.7 dm3 mol−1 =» 126 «dm3» ✓
Award [2] for correct final answer.
Accept method using pV = nRT to obtain the volume with p as either 100 kPa (126 dm3) or 101.3 kPa (125 dm3).
Do not penalize use of 22.4 dm3 mol–1 to obtain the volume (124 dm3).
«ΔH =» (−635 «kJ» – 393.5 «kJ») – (−1207 «kJ») ✓
«ΔH = + » 179 «kJ» ✓
Award [2] for correct final answer.
Award [1 max] for −179 kJ.
Ignore an extra step to determine total enthalpy change in kJ: 179 kJ mol-1 x 5.55 mol = 993 kJ.
Award [2] for an answer in the range 990 - 993« kJ».
«ΔS = (40 J K−1 + 214 J K−1) − (93 J K−1) =» 161 «J K−1» ✓
Ignore an extra step to determine total entropy change in JK–1: 161 J mol–1K–1 x 5.55 mol = 894 «J mol–1K–1»
Award [1] for 894 «J mol–1K–1».
«spontaneous» if ΔG = ΔH − TΔS < 0
OR
ΔH < TΔS ✓
«T >=» 1112 «K» ✓
Award [2] for correct final answer.
Accept “1056 K” if both of the incorrect values are used to solve the problem.
Do not award M2 for any negative T value.
endothermic sketch ✓
x-axis labelled “extent of reaction/progress of reaction/reaction coordinate/reaction pathway” AND y-axis labelled “potential energy/energy/enthalpy✓
activation energy/Ea ✓
Do not accept “time” for x-axis.
ΔH same AND lower Ea ✓
Ca(OH)2 (aq) + 2HCl (aq) → 2H2O (l) + CaCl2 (aq) ✓
«nHCl = 0.0350 dm3 × 0.025 mol dm−3 =» 0.00088 «mol»
OR
nCa(OH)2 = nHCl/0.00044 «mol» ✓
«V = =» 0.029 «dm3» ✓
Award [2] for correct final answer.
Award [1 max] for 0.058 «dm3».
Alternative 1:
[OH−] = « 2 × 2.33 × 10−2 mol dm−3 =» 0.0466 «mol dm−3» ✓
«[H+] = = 2.15 × 10−13 mol dm−3»
pH = « −log (2.15 × 10−13) =» 12.668 ✓
Alternative 2:
[OH−] =« 2 × 2.33 × 10−2 mol dm−3 =» 0.0466 «mol dm−3» ✓
«pOH = −log (0.0466) = 1.332»
pH = «14.000 – pOH = 14.000 – 1.332 =» 12.668 ✓
Award [2] for correct final answer.
Award [1 max] for pH =12.367.
«nCa(OH)2 = 2.41 dm3 × 2.33 × 10−2 mol dm−3 =» 0.0562 «mol» AND
«nCO2 ==» 0.0330 «mol» ✓
«CO2 is the limiting reactant»
«mCaCO3 = 0.0330 mol × 100.09 g mol−1 =» 3.30 «g» ✓
Only award ECF for M2 if limiting reagent is used.
Accept answers in the range 3.30 - 3.35 «g».
« × 100 =» 86.4 «%» ✓
Accept answers in the range 86.1-86.4 «%».
Accept “71.3 %” for using the incorrect given value of 4.00 g.
«add» Ca(OH)2/CaCO3/CaO AND to «acidic» water/river/lake/soil
OR
«use» Ca(OH)2/CaCO3/CaO in scrubbers «to prevent release of acidic pollution» ✓
Accept any correct name for any of the calcium compounds listed.
Examiners report
Enthalpy changes depend on the number and type of bonds broken and formed.
Enthalpy changes depend on the number and type of bonds broken and formed.
The table lists the standard enthalpies of formation, , for some of the species in the reaction above.

Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
Outline why no value is listed for H2(g).
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
The table lists standard entropy, SΘ, values.

Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
Determine the temperature, in K, above which the reaction becomes spontaneous.
Markscheme
bonds broken: 4(C–H) + 2(H–O)/4(414) + 2(463)/2582 «kJ»
bonds made: 3(H–H) + C≡O/3(436) + 1077/2385 «kJ»
ΔH «= ΣBE(bonds broken) – ΣBE(bonds made) = 2582 – 2385» = «+» 197 «kJ»
Award [3] for correct final answer.
Award [2 max] for –197 «kJ».
[3 marks]
for any element = 0 «by definition»
OR
no energy required to form an element «in its stable form» from itself
[1 mark]
ΔHΘ « = (products) – (reactants) = –111 + 0 – [–74.0 + (–242)]»
= «+» 205 «kJ»
[1 mark]
«ΔSΘ = ΣSΘproducts – ΣSΘreactants = 198 + 3 × 131 – (186 + 189) =» «+» 216 «J K–1»
[1 mark]
«ΔGΘ = ΔHΘ – TΔSΘ = 205 kJ – 298 K × kJ K–1 =» «+» 141 «kJ»
[1 mark]
«ΔHΘ = TΔSΘ»
«»
«T =» 949 «K»
Do not award a mark for negative value of T.
[1 mark]
Examiners report
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
Markscheme
absorbs UV/ultraviolet light «of longer wavelength than absorbed by O2» [✔]
NO (g) + O3 (g) → NO2 (g) + O2 (g) [✔]
NO2 (g) + O3 (g) → NO (g) + 2O2 (g) [✔]
Note: Ignore radical signs.
Accept equilibrium arrows.
Award [1 max] for NO2 (g) + O (g) → NO (g) + O2 (g).
mass spectrometry/MS [✔]
« =» 14.02 [✔]
«Mr = (14.02 × 2) + 16.00 =» 44.04 [✔]
Any two:
same AND have same nuclear charge /number of protons/Zeff [✔]
same AND neutrons do not affect attraction/ionization energy/Zeff
OR
same AND neutrons have no charge [✔]
same AND same attraction for «outer» electrons [✔]
same AND have same electronic configuration/shielding [✔]
Note: Accept “almost the same”.
“Same” only needs to be stated once.
Nitrogen and carbon:
N has greater nuclear charge/«one» more proton «and electrons both lost from singly filled p-orbitals» [✔]
Nitrogen and oxygen:
O has a doubly filled «p-»orbital
OR
N has only singly occupied «p-»orbitals [✔]
Note: Accept “greater e– - e- repulsion in O” or “lower e– - e- repulsion in N”.
Accept box annotation of electrons for M2.
delocalization
OR
delocalized π-electrons [✔]
Note: Accept “resonance”.
linear AND 2 electron domains
OR
linear AND 2 regions of electron density [✔]
Note: Accept “two bonds AND no lone pairs” for reason.
sp [✔]
Examiners report
Candidates sometimes failed to identify how ozone works in chemical terms, referring to protects/deflects, i.e., the consequence rather than the mechanism.
Many candidates recalled the first equation for NO catalyzed decomposition of ozone only. Some considered other radical species.
All candidates, with very few exceptions, answered this correctly.
Most candidates were able to calculate the accurate mass of N2O, though quite a few candidates just calculated the mass of N and didn’t apply it to N2O, losing an accessible mark.
Many students realized that neutrons had no charge and could not affect IE significantly, but many others struggled a lot with this question since they considered that 15N would have a higher IE because they considered the greater mass of the nucleus would result in an increase of attraction of the electrons.
Mixed responses here; the explanation of higher IE for N with respect to C was less well explained, though it should have been the easiest. It was good to see that most candidates could explain the difference in IE of N and O, either mentioning paired/unpaired electrons or drawing box diagrams.
Most candidates identified resonance for this given Lewis representation.
Though quite a number of candidates suggested a linear shape correctly, they often failed to give a complete correct explanation, just mentioning the absence of lone pairs but not two bonds, instead of referring to electron domains.
Hybridisation of the N atom was correct in most cases.
This question is about sodium and its compounds.
The Born-Haber cycle for sodium oxide is shown (not to scale).
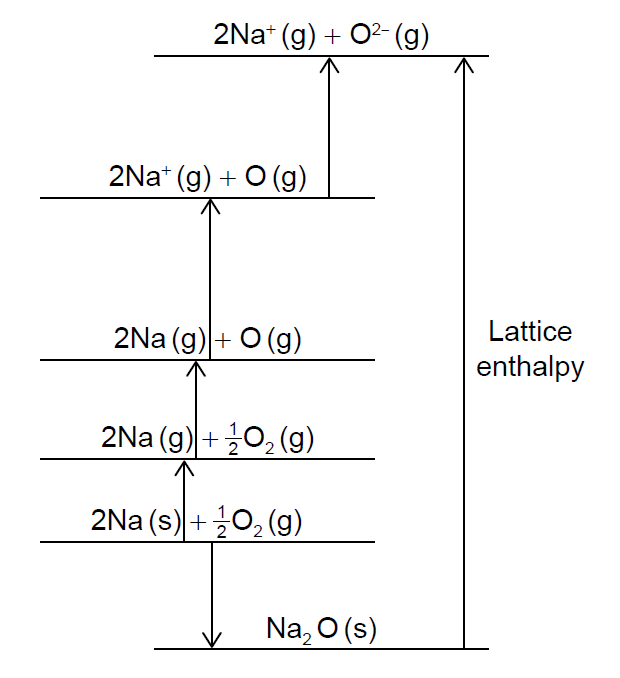
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Plot the relative values of the first four ionization energies of sodium.
Outline why the alkali metals (group 1) have similar chemical properties.
Describe the structure and bonding in solid sodium oxide.
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1
O2(g) → O2- (g):
Na (s) → Na+ (g):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.)
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00g of sodium oxide produces 5.50g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
Outline why a real gas differs from ideal behaviour at low temperature and high pressure.
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Markscheme
[✔]
Notes: Accept curve showing general trend.
Award mark only if the energy difference between the first two points is larger than that between points 2/3 and 3/4.
same number of electrons in outer shell
OR
all are s1 [✔]
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions» [✔]
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Na+ and O2− ions [✔]
Note: Do not accept “ionic” without description.
O2(g) → O2- (g)
«ΔHatomisation (O) + 1st EA + 2nd EA = 249 k Jmol−1 − 141 kJmol−1 + 753 kJmol−1 =» «+»861 «kJmol−1» [✔]
Na (s) → Na+ (g)
«ΔHatomisation (Na) + 1st IE = 107 kJmol−1 + 496 kJmol−1 =» «+»603 «kJmol−1» [✔]
lattice enthalpy = 861 «kJ mol−1» + 2 × 603 «kJ mol−1» −(−414 «kJ mol−1») [✔]
«= +» 2481 «kJ mol−1» [✔]
Note: Award [2] for correct final answer.
If given values are used:
M1: lattice enthalpy = 850 «kJ mol−1» +
2 × 600 «kJ mol−1» −(−414 «kJ mol−1»)
M2: «= +» 2464 «kJ mol−1»
K+ ion is larger than Na+
OR
smaller attractive force because of greater distance between ion «centres» [✔]
Sodium oxide:
Na2O(s) + H2O(l) → 2NaOH (aq) [✔]
Phosphorus(V) oxide:
P4O10 (s) + 6H2O(l) → 4H3PO4 (aq) [✔]
Differentiation:
NaOH/product of Na2O is alkaline/basic/pH > 7 AND H3PO4/product of P4O10 is acidic/pH < 7 [✔]
n(Na2O2) theoretical yield «= » = 0.0807/8.07 × 10−2 «mol»
OR
mass of Na2O2 theoretical yield «= × 77.98 gmol−1» = 6.291 «g» [✔]
% yield «= × 100» OR « × 100» = 87.4 «%» [✔]
Note: Award [2] for correct final answer.
∑ΔHf products = 2 × (−1130.7) / −2261.4 «kJ» [✔]
∑ΔHf reactants = 2 × (−510.9) + 2 × (−393.5) / −1808.8 «kJ» [✔]
ΔH = «∑ΔHf products − ∑ΔHf reactants = −2261.4 −(−1808.8) =» −452.6 «kJ» [✔]
Note: Award [3] for correct final answer.
Award [2 max] for “+ 452.6 «kJ»”.
only valid for covalent bonds
OR
only valid in gaseous state [✔]
bond in O3 has lower enthalpy AND bond order is 1.5 «not 2» [✔]
Note: Accept “bond in ozone is longer”.
Any one of:
finite volume of particles «requires adjustment to volume of gas» [✔]
short-range attractive forces «overcomes low kinetic energy» [✔]
NaOH [✔]
IV [✔]
Examiners report
Generally well done with a correct plot of ionization energies.
The majority answered correctly stating same number of valence electrons as the reason. Some candidates stated same size or similar ionization energy but the majority scored well.
Many candidates lost one or two marks for missing “electrostatic forces” between “oppositely charged ions”, or “lattice”. Some candidates’ answers referred to covalent bonds and shapes of molecules.
Good performance with typical error being in the calculation for the first equation, ½O2 (g) → O2− (g), where the value for the first electron affinity of oxygen was left out.
Many candidates earned some credit for ECF based on (d)(i).
Average performance with answers using atomic size rather than ionic size or making reference to electronegativities of K and Na.
An average of 1.1 out of 3 earned here. Many candidates could write a balanced equation for the reaction of sodium oxide with water but not phosphorus(V) oxide. Mediocre performance in identifying the acid/base nature of the solutions formed.
The majority earned one or two marks in finding a % yield.
The average was 2.2 out 3 for this question on enthalpy of formation. Enthalpy calculations were generally well done.
The majority of candidates referred to “bond enthalpy values are average”, rather than not valid for solids or only used for gases.
Some candidates recognized that ozone had a resonance structure but then only compared bond length between ozone and oxygen rather than bond enthalpy.
Few candidates could distinguish the cause for difference in behaviour between real and ideal gases at low temperature or high pressure. Many answers were based on increase in number of collisions or faster rate or movement of gas particles.
Na2O was a common formula in many candidates’ answers for the product of the reaction of sodium peroxide with water.
The vast majority of candidates could correctly state the oxidation number of carbon in sodium carbonate.
Ethane-1,2-diol, HOCH2CH2OH, reacts with thionyl chloride, SOCl2, according to the reaction below.
HOCH2CH2OH (l) + 2SOCl2 (l) → ClCH2CH2Cl (l) + 2SO2 (g) + 2HCl (g)
Calculate the standard enthalpy change for this reaction using the following data.
Calculate the standard entropy change for this reaction using the following data.
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
Markscheme
ΔHθ = [–165.2 + 2(–296.9) + 2(–92.3)] – [–454.7 + 2(–245.7)]
«ΔHθ = +»2.5 «kJ»
Award [2] for correct final answer.
Award [1] for –2.5 «kJ».
Do not accept ECF for M2 if more than one error in M1.
«ΔSθ = [208.5 + 2(248.1) + 2(186.8)] – [166.9 + 2(278.6)]»
«ΔSθ = +» 354.2 «J K–1 mol–1»
«3 moles of» liquid to «4 moles of» gas
OR
«large» positive ΔS
OR
«large» increase in entropy
TΔS > ΔH «at the reaction temperature»
Examiners report
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Markscheme
Accept any diagram with each P joined to the other three.
Accept any combination of dots, crosses and lines.
P4 (s) + 6Cl2 (g) → 4PCl3 (l) ✔
Electron domain geometry: tetrahedral ✔
Molecular geometry: trigonal pyramidal ✔
Bond angle: 100«°» ✔
Accept any value or range within the range 91−108«°» for M3.
PCl5 is non-polar:
symmetrical
OR
dipoles cancel ✔
PCl4F is polar:
P–Cl has a different bond polarity than P–F ✔
non-symmetrical «dipoles»
OR
dipoles do not cancel ✔
Accept F more electronegative than/different electronegativity to Cl for M2.
«−398.9 kJ mol−1 − (−306.4 kJ mol−1) =» −92.5 «kJ mol−1» ✔
«ΔS = 364.5 J K–1 mol–1 – (311.7 J K–1 mol–1 + 223.0 J K–1 mol–1)=» –170.2 «J K–1 mol–1» ✔
«ΔS =» –0.1702 «kJ mol–1 K–1»
OR
298 «K» ✔
«ΔG = –92.5 kJ mol–1 – (298 K × –0.1702 kJ mol–1 K–1) =» –41.8 «kJ mol–1» ✔
Award [2] for correct final answer.
If –87.6 and -150.5 are used then –42.8.
«ΔG = –41.8 kJ mol–1 = × 298 K × lnK»
OR
«ΔG = –41800 J mol–1 = –8.31 J mol–1 K–1 × 298 K × lnK»
«lnK = =» 16.9 ✔
«K = e16.9 =» 2.19 × 107 ✔
Award [2] for correct final answer.
Accept range of 1.80 × 106–2.60 × 107.
If –43.5 is used then 4.25 × 107.
«Kc =» ✔
«shifts» left/towards reactants AND «forward reaction is» exothermic/ΔH is negative ✔
Examiners report
Organic chemistry can be used to synthesize a variety of products.
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
State the hybridization of the carbon I and II atoms in but-2-ene.
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly arrows.
Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
Deduce two features of this molecule that can be obtained from the mass spectrum. Use section 28 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Identify the bond responsible for the absorption at A in the infrared spectrum. Use section 26 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet.
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
Outline how two enantiomers can be distinguished using a polarimeter.
Markscheme
Penalize missing hydrogens in displayed structural formulas once only.
Accept condensed structural formulas: CH3CH(OH)CH2CH3 / CH3CH2CH2CH3 or skeletal structures.
Bonds broken:
2(C–C) + 1(C=C) + 8(C–H) + 6O=O / 2(346) + 1(614) + 8(414) + 6(498) / 7606 «kJ» ✓
Bonds formed:
8(C=O) + 8(O–H) / 8(804) + 8(463) / 10 136 «kJ» ✓
Enthalpy change:
«Bonds broken – Bonds formed = 7606 kJ – 10 136 kJ =» –2530 «kJ» ✓
Award [3] for correct final answer.
Award [2 max] for «+» 2530 «kJ».
Sigma (σ):
Accept any diagram showing end to end/direct overlap of atomic/hybridized orbitals and electron density concentrated between nuclei.
Pi (π):
Accept any diagram showing sideways overlap of unhybridized p/atomic orbitals and electron density above and below plane of bond axis.
Alternative 1
Penalize incorrect bond e.g., -CH-H3C or –CH3C only once in the paper.
Alternative 2
curly arrow going from C=C to H of HBr AND curly arrow showing Br leaving ✓
representation of carbocation ✓
curly arrow going from lone pair/negative charge on Br− to C+ ✓
«2-bromo-2-methylbutane involves» formation of more stable «tertiary» carbocation/intermediate
OR
«2-bromo-3-methylbutane involves» formation of less stable «secondary» carbocation/intermediate ✓
«intermediate» more stable due to «increased positive» inductive/electron-releasing effect of extra –R/alkyl group/–CH3/methyl ✓
Do not award marks for quoting Markovnikov’s rule without any explanation.
m/z 58:
molar/«relative» molecular mass/weight/Mr «is 58 g mol−1/58» ✓
m/z 43:
«loses» methyl/CH3 «fragment»
OR
COCH3+ «fragment» ✓
Do not penalize missing charge on the fragments.
Accept molecular ion «peak»/ CH3COCH3+/C3H6O+.
Accept any C2H3O+ fragment/ CH3CH2CH2+/C3H7+.
C=O ✓
Accept carbonyl/C=C.
Information deduced from 1H NMR:
«one signal indicates» one hydrogen environment/symmetrical structure
OR
«chemical shift of 2.2 indicates» H on C next to carbonyl ✓
Compound:
propanone/CH3COCH3 ✓
Accept “one type of hydrogen”.
Accept .
enantiomers rotate «plane of» plane-polarized light ✓
equal degrees/angles/amounts AND opposite directions/rotation ✓
Accept “optical isomers” for “enantiomers”.
Examiners report
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.
Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
Deduce the half-equation for the formation of the gas identified in (c)(iii).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
Comment on the spontaneity of the disproportionation reaction at 298 K.
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the disproportionation reaction at 298 K.
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
Deduce why the Cu(I) solution is colourless.
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
Markscheme
[Ar] 3d10
OR
1s2 2s2 2p6 3s2 3p6 3d10 ✔
ΔHθ = ΣΔHθf (products) − ΣΔHθf (reactants) ✔
ΔHθ = 2(−241.8 «kJ mol−1») − 4(−92.3 «kJ mol−1») = −114.4 «kJ» ✔
NOTE: Award [2] for correct final answer.
Ea (cat) to the left of Ea ✔
peak lower AND Ea (cat) smaller ✔
«catalyst provides an» alternative pathway ✔
«with» lower Ea
OR
higher proportion of/more particles with «kinetic» E ≥ Ea(cat) «than Ea» ✔
mass of H2O = «18.360 g – 17.917 g =» 0.443 «g» AND mass of CuCl2 = «17.917 g – 16.221 g =» 1.696 «g» ✔
moles of H2O = «=» 0.0246 «mol»
OR
moles of CuCl2 =«= » 0.0126 «mol» ✔
«water : copper(II) chloride = 1.95 : 1»
« =» 2 ✔
NOTE: Accept « =» 1.95.
NOTE: Award [3] for correct final answer.
Wires:
«delocalized» electrons «flow» ✔
Electrolyte:
«mobile» ions «flow» ✔
2Cl− → Cl2 (g) + 2e−
OR
Cl− → Cl2 (g) + e− ✔
NOTE: Accept e for e−.
«electrode» 3 AND oxygen/O2 ✔
NOTE: Accept chlorine/Cl2.
2H2O (l) → 4H+ (aq) + O2 (g) + 4e– ✔
NOTE: Accept 2Cl– (aq) → Cl2 (g) + 2e–.
Accept 4OH− → 2H2O + O2 + 4e−
enthalpy of solution = lattice enthalpy + enthalpies of hydration «of Cu2+ and Cl−» ✔
«+2824 kJ mol–1 − 2161 kJ mol–1 − 2(359 kJ mol–1) =» −55 «kJ mol–1» ✔
NOTE: Accept enthalpy cycle.
Award [2] for correct final answer.
Eθ = «+0.52 – 0.15 = +» 0.37 «V» ✔
spontaneous AND Eθ positive ✔
ΔGθ = «−nFE = −1 mol × 96 500 C Mol–1 × 0.37 V=» −36 000 J/−36 kJ ✔
NOTE: Accept “−18 kJ mol–1 «per mole of Cu+»”.
Do not accept values of n other than 1.
Apply SF in this question.
Accept J/kJ or J mol−1/kJ mol−1 for units.
2 mol (aq) → 1 mol (aq) AND decreases ✔
NOTE: Accept “solid formed from aqueous solution AND decreases”.
Do not accept 2 mol → 1 mol without (aq).
ΔGθ < 0 AND ΔSθ < 0 AND ΔHθ < 0
OR
ΔGθ + TΔSθ < 0 AND ΔHθ < 0 ✔
TΔS more negative «reducing spontaneity» AND stability increases ✔
NOTE: Accept calculation showing non-spontaneity at 433 K.
«ligands cause» d-orbitals «to» split ✔
light absorbed as electrons transit to higher energy level «in d–d transitions»
OR
light absorbed as electrons promoted ✔
energy gap corresponds to «orange» light in visible region of spectrum ✔
colour observed is complementary ✔
full «3»d sub-level/orbitals
OR
no d–d transition possible «and therefore no colour» ✔
octahedral AND 90° «180° for axial» ✔
NOTE: Accept square-based bi-pyramid.
Any two of:
ligand/chloride ion Lewis base AND donates e-pair ✔
not Brønsted–Lowry base AND does not accept proton/H+ ✔
Lewis definition extends/broader than Brønsted–Lowry definition ✔
Examiners report
3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm−3 copper(II) sulfate solution. The following reaction occurs:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Determine the limiting reactant showing your working.
The mass of copper obtained experimentally was 0.872 g. Calculate the percentage yield of copper.
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
State another assumption you made in (b)(i).
The only significant uncertainty is in the temperature measurement.
Determine the absolute uncertainty in the calculated value of ΔH if the uncertainty in the temperature rise was ±0.2 °C.
Sketch a graph of the concentration of iron(II) sulfate, FeSO4, against time as the reaction proceeds.
Outline how the initial rate of reaction can be determined from the graph in part (c)(i).
Explain, using the collision theory, why replacing the iron powder with a piece of iron of the same mass slows down the rate of the reaction.
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of the data booklet.
Markscheme
nCuSO4 «= 0.0800 dm3 × 0.200 mol dm–3» = 0.0160 mol AND
nFe «» = 0.0584 mol ✔
CuSO4 is the limiting reactant ✔
Do not award M2 if mole calculation is not shown.
ALTERNATIVE 1:
«0.0160 mol × 63.55 g mol–1 =» 1.02 «g» ✔
«» 85.5 «%» ✔
ALTERNATIVE 2:
«» 0.0137 «mol» ✔
«» 85.6 «%» ✔
Accept answers in the range 85–86 %.
Award [2] for correct final answer.
ALTERNATIVE 1:
q = «80.0 g × 4.18 J g–1 K–1 × 7.5 K =» 2.5 × 103 «J»/2.5 «kJ» ✔
«per mol of CuSO4 = kJ mol–1»
«for the reaction» ΔH = –1.6 × 102 «kJ» ✔
ALTERNATIVE 2:
q = «80.0 g × 4.18 J g–1 K–1 × 7.5 K =» 2.5 × 103 «J»/2.5 «kJ» ✔
«nCu = = 0.0137 mol»
«per mol of CuSO4 = kJ mol–1»
«for the reaction» ΔH = –1.8 × 102 «kJ» ✔
Award [2] for correct final answer.
density «of solution» is 1.00 g cm−3
OR
specific heat capacity «of solution» is 4.18 J g−1 K−1/that of «pure» water
OR
reaction goes to completion
OR
iron/CuSO4 does not react with other substances ✔
The mark for “reaction goes to completion” can only be awarded if 0.0160 mol was used in part (b)(i).
Do not accept “heat loss”.
ALTERNATIVE 1:
«» 3 %/0.03 ✔
«0.03 × 160 kJ» = «±» 5 «kJ» ✔
ALTERNATIVE 2:
«» 3 %/0.03 ✔
«0.03 × 180 kJ» = «±» 5 «kJ» ✔
Accept values in the range 4.1–5.5 «kJ».
Award [2] for correct final answer.
initial concentration is zero AND concentration increases with time ✔
decreasing gradient as reaction proceeds ✔
«draw a» tangent to the curve at time = 0 ✔
«rate equals» gradient/slope «of the tangent» ✔
Accept suitable diagram.
piece has smaller surface area ✔
lower frequency of collisions
OR
fewer collisions per second/unit time ✔
Accept “chance/probability” instead of “frequency”.
Do not accept just “fewer collisions”.
Anode (positive electrode):
2H2O (l) → O2 (g) + 4H+ (aq) + 4e– ✔
Cathode (negative electrode):
2H2O (l) + 2e– → H2 (g) + 2OH– (aq)
OR
2H+ (aq) + 2e– → H2 (g) ✔
Accept “4OH– (aq) → O2 (g) + 2H2O (l) + 4e–” OR “Fe2+ (aq) → Fe3+ (aq) + e–” for M1.
Accept “Fe2+ (aq) + 2e– → Fe (s)” OR “SO42- (aq) 4H+ (aq) + 2e– → 2H2SO3(aq) + H2O (l)”
for M2.
Examiners report
Propene is an important starting material for many products. The following shows some compounds which can be made from propene, C3H6.
Propene (C3H6) → C3H7Cl → C3H8O → C3H6O
Consider the conversion of propene to C3H7Cl.
An experiment was carried out to determine the order of reaction between one of the isomers of C3H7Cl and aqueous sodium hydroxide. The following results were obtained.
State the type of reaction.
State the IUPAC name of the major product.
Outline why it is the major product.
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
Determine the rate expression from the results, explaining your method.
Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium hydroxide.
Sketch the mechanism using curly arrows to represent the movement of electrons.
Write an equation for the complete combustion of the compound C3H8O formed in (a)(iv).
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
State the reagents for the conversion of the compound C3H8O formed in (a)(iv) into C3H6O.
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
Explain why the 1H NMR spectrum of C3H6O, produced in (d)(i), shows only one signal.
Propene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
Markscheme
«electrophilic» addition ✔
NOTE: Do not accept “nucleophilic addition” or “free radical addition”.
Do not accept “halogenation”.
2-chloropropane ✔
secondary carbocation/carbonium «ion» is more stable
OR
carbocation/carbonium «ion» stabilized by two/more alkyl groups ✔
CH3CHClCH3 (l) + OH− (aq) → CH3CH(OH)CH3 (aq) + Cl− (aq)
OR
CH3CHClCH3 (l) + NaOH (aq) → CH3CH(OH)CH3 (aq) + NaCl (aq) ✔
Rate = k [C3H7Cl] [OH−] ✔
«[OH−] held constant and» [C3H7Cl] triples AND rate triples «so first order wrt C3H7Cl» ✔
[C3H7Cl] doubles AND [OH−] doubles AND rate quadruples «so first order wrt OH−» ✔
SN2 ✔
NOTE: Accept ‘bimolecular nucleophilic substitution.’
curly arrow going from lone pair on O/negative charge on OH– to C ✔
curly arrow showing C–Cl bond breaking ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
formation of CH3CH(OH)CH3 AND Cl– ✔
NOTE: Do not allow arrow originating on H in OH–.
Allow curly arrow going from bond between C and Cl to Cl in either reactant or transition state.
Do not award M3 if OH–C bond is represented.
Accept formation of NaCl instead of Cl–.
2C3H8O (l) + 9O2 (g) → 6CO2 (g) + 8H2O (g)
OR
C3H8O (l) + 4.5O2 (g) → 3CO2 (g) + 4H2O (g) ✔
bonds broken:
7(C–H) + C–O + O–H + 2(C–C) + 4.5(O=O)
OR
7(414 «kJ mol−1») + 358 «kJ mol−1» + 463 «kJ mol−1» + 2(346 «kJ mol−1») + 4.5(498 «kJ mol−1») / 6652 «kJ» ✔
bonds formed:
6(C=O) + 8(O–H)
OR
6(804 «kJ mol−1») + 8(463 «kJ mol−1») / 8528 «kJ» ✔
«ΔH = bonds broken − bonds formed = 6652 – 8528 =» −1876 «kJ mol−1» ✔
NOTE: Award [3] for correct final answer.
K2Cr2O7/Cr2O72–/«potassium» dichromate «(VI)» AND acidified/H+
OR
«acidified potassium» manganate(VII) / «H+ and» KMnO4 / «H+ and» MnO4– ✔
NOTE: Accept “H2SO4” or “H3PO4” for “H+”.
Do not accept HCl.
Accept “permanganate” for “manganate(VII)”.
C3H8O/propan-2-ol: hydrogen-bonding AND C3H6O/propanone: no hydrogen bonding/«only» dipole–dipole/dispersion forces ✔
hydrogen bonding stronger «than dipole–dipole» ✔
only one hydrogen environment
OR
methyl groups symmetrical «around carbonyl group» ✔
NOTE: Accept “all hydrogens belong to methyl groups «which are in identical positions»”.
✔
NOTE: Continuation bonds must be shown.
Methyl groups may be drawn on opposite sides of the chain or head to tail.
Ignore square brackets and “n”.
Examiners report
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.
Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
Markscheme
1:2 ✔
Accept 2 Fe3+: 1 Fe2+
Do not accept 2:1 only
mass «spectroscopy»/MS ✔
Award [1 max] for 4 correct values.
specific heat capacity « = » = 0.45 «J g−1 K−1» ✔
Equation:
2Fe3+(aq) + Fe(s) → 3Fe2+(aq) ✔
Cell potential:
«+0.77 V − (−0.45 V) = +»1.22 «V» ✔
Do not accept reverse reaction or equilibrium arrow.
Do not accept negative value for M2.
left electrode/anode labelled zinc/Zn AND right electrode/cathode labelled iron/Fe ✔
electrolyte labelled as «aqueous» zinc salt/Zn2+ ✔
Accept an inert conductor for the anode.
Accept specific zinc salts such as ZnSO4.
« Zn2+» has a full d-shell
OR
does not form « ions with» an incomplete d-shell ✔
Do not accept “Zn is not a transition metal”.
Do not accept zinc atoms for zinc ions.
ligands donate pairs of electrons to metal ions
OR
forms coordinate covalent/dative bond✔
ligands are Lewis bases
AND
metal «ions» are Lewis acids ✔
Examiners report
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
Predict the 1HNMR data for ethanedioic acid and ethane-1,2-diol by completing the table.
Markscheme
i
«ΔH = Σ ΔHf products – ΣΔHf reactants = –454.8 kJ mol-1 – 2(–110.5 kJ mol-1) =» –233.8 «kJ»
ii
in (a)(iii) gas is formed and in (b)(i) liquid is formed
OR
products are in different states
OR
conversion of gas to liquid is exothermic
OR
conversion of liquid to gas is endothermic
OR
enthalpy of vapourisation needs to be taken into account
Accept product is «now» a liquid.
Accept answers referring to bond enthalpies being means/averages.
iii
«ΔS is negative because five mols of» gases becomes «one mol of» liquid
OR
increase in complexity of product «compared to reactants»
OR
product more ordered «than reactants»
Accept “fewer moles of gas” but not “fewer molecules”.
iv
ΔS = «kJ K-1»
ΔG = –233.8 kJ – (298 K kJ K-1) = –49.0 «kJ»
Award [2] for correct final answer.
Award [1 max] for «+»185 × 103.
If –244.0 kJ used, answer is:
ΔG = –244.0 kJ – (298 K kJ K-1) = –59.2 «kJ»
Award [2] for correct final answer.
v
increasing T makes ΔG larger/more positive/less negative
OR
–TΔS will increase
Accept “none/no splitting” for singlet.
Examiners report
A student titrated two acids, hydrochloric acid, HCl (aq) and ethanoic acid, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine their concentration. The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of each acid.
Using the graph, estimate the initial temperature of the solutions.
Determine the maximum temperature reached in each experiment by analysing the graph.
Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
Markscheme
21.4 °C
Accept values in the range of 21.2 to 21.6 °C.
Accept two different values for the two solutions from within range.
HCl: 30.4 «°C»
Accept range 30.2 to 30.6 °C.
CH3COOH: 29.0 «°C»
Accept range 28.8 to 29.2 °C.
CH3COOH is weak acid/partially ionised
energy used to ionize weak acid «before reaction with NaOH can occur»